Saturated Steam Table - Thermodynamics
Thermodynamics Directory | Heat Transfer Directory
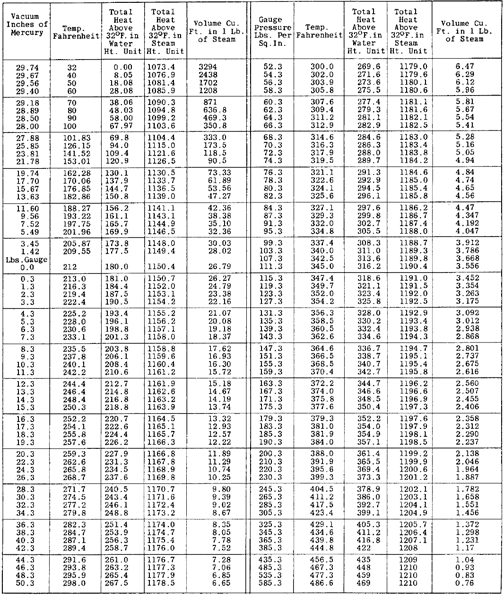
Saturated Steam Table
Related Resources:
Saturated steam is, in contrast to superheated steam, steam that is in equilibrium with heated water at the same pressure, i.e., it has not been heated past the boiling point for that pressure. If saturated steam is reduced in temperature (while retaining its pressure) it will condense to produce water droplets, even if it is still considerably above the boiling point of 100 deg. C at standard pressure. These condensation droplets are a cause of damage to steam turbine blades, the reason why such turbines rely on a supply of dry, superheated steam.