Related Resources: heat transfer
Water Boiling Graph Curve at 1 Atmosphere
Heat Transfer Engineering
Thermodynamics
Engineering Physics
Typical Water Boiling Graph Curve at 1 Atmosphere
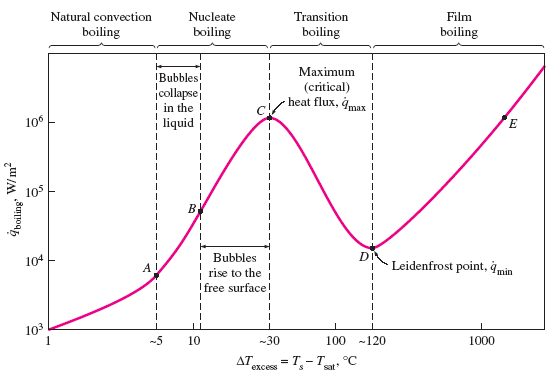
The stages of boiling are:
- Natural Convection Boiling (to Point A on the Boiling Curve) No bubbles forming on the heating surface until the liquid is heated a few degrees above the saturation temperature (about 2 to 6°C for water).
- Nucleate Boiling (between Points A and C) The first bubbles start forming at point A of the boiling curve at various preferential sites on the heating surface. The bubbles form at an increasing rate at an increasing number of nucleation sites as we move along the boiling curve toward point C. The nucleate boiling regime can be separated into two distinct regions. In region A–B, isolated bubbles are formed at various preferential nucleation sites on the heated surface. But these bubbles are dissipated in the liquid shortly after they separate from the surface. The space vacated by the rising bubbles is filled by the liquid in the vicinity of the heater surface, and the process is repeated. The stirring and agitation caused by the entrainment of the liquid to the heater surface is primarily responsible for the increased heat transfer coefficient and heat flux in this region of nucleate boiling. In region B–C, the heater temperature is further increased, and bubbles form at such great rates at such a large number of nucleation sites that they form numerous continuous columns of vapor in the liquid. These bubbles move all the way up to the free surface, where they break up and release their vapor content. The large heat fluxes obtainable in this region are caused by the combined effect of liquid entrainment and evaporation.
- Transition Boiling (between Points C and D on the
Boiling Curve)
As the heater temperature and thus the ΔTexcess is increased past point C, the heat flux decreases. This is because a large fraction of the heater surface is covered by a vapor film, which acts as an insulation due to the low thermal conductivity of the vapor relative to that of the liquid. In the transition boiling regime, both nucleate and film boiling partially occur. Nucleate boiling at point C is completely replaced by film boiling at point D. Operation in the transition boiling regime, which is also called the unstable film boiling regime, is avoided in practice. For water, transition boiling occurs over the excess temperature range from about 30°C to about 120°C. - Film Boiling (beyond Point D) In this region the heater surface is completely covered by a continuous stable vapor film. Point D, where the heat flux reaches a minimum, is called the Leidenfrost point, in honor of J. C. Leidenfrost, who observed in 1756 that liquid droplets on a very hot surface jump around and slowly boil away. The presence of a vapor film between the heater surface and the liquid is responsible for the low heat transfer rates in the film boiling region. The heat transfer rate increases with increasing excess temperature as a result of heat transfer from the heated surface to the liquid through the vapor film by radiation, which becomes significant at high temperatures.