Related Resources: fluid flow
Flash Point Liquids, Gases, Vapors and Solvents
Flash Point of Flammable Gases, Vapors and Solvents
Flash Point of Liquids
A flammable liquid’s vapor pressure and volatility or rate of evaporation determine its ability to form an explosive mixture. These properties can be expressed by the flash point, which is the temperature to which a flammable liquid must be heated to produce a flash when a small flame is passed across the surface of the liquid. Depending on the test methods, either the open- or closed-cup flash point may be listed. The higher the flash point, the more safely the liquid can be handled. Liquids with flash points higher than 100°F are called combustible, whereas those under 100°F are described as flammable. Those with flash points less than 70°F should be regarded as highly flammable.
Table 1.0 Flammable Limits of Some Gases and Vapors
| Gas or Vapor |
Flash Point,* °F
|
Flammable Limits, % by Volume
|
|
|
Lower
|
Upper
|
||
|
Acetone |
0
|
2.5
|
12.8
|
| Ammonia |
Gas
|
15
|
28
|
| Benzene (benzol) |
12
|
1.2
|
7.8
|
| n-Butane |
–26
|
1.9
|
8.5
|
| Carbon disulfide |
–22
|
1.3
|
50
|
| Carbon monoxide |
Gas
|
12.5
|
74
|
| 1,2-Dichloroethylene |
36
|
5.6
|
12.8
|
| Diethylether |
–49
|
1.9
|
36
|
| Ethyl alcohol |
55
|
3.3
|
19
|
| Ethylene |
Gas
|
2.7
|
36
|
| Gasoline |
–45
|
1.4
|
7.6
|
| Hydrogen |
Gas
|
4.0
|
75
|
| Hydrogen sulfide |
Gas
|
4.3
|
44
|
| Isopropyl alcohol |
53 .
|
2.0
|
12.7
|
| Methyl alcohol |
52
|
6.0
|
36
|
| Methyl ethyl ketone |
16
|
1.4
|
11.4
|
| Natural gas (variable) |
Gas
|
3.8 to 6.5
|
13 to 17
|
| Naphtha |
Less than 0
|
1.1
|
5.9
|
| Propane |
Gas
|
2.1
|
9.5
|
| Toluene (toluol) |
40
|
0.1
|
7.1
|
| o-Xylene |
90
|
0.9
|
6.7
|
Table 2.0 . Flash Point Data for Solvents
| Chemical | Flash Point | Boiling Point | NFPA Class | ||
| ° F | °C | °F | °C | ||
| Propane | (157) | (105) | (44) | (42) | IA |
| Pentane | (57) | (49) | 97 | 36 | IA |
| Ethyl ether | (49) | (45) | 95 | 35 | IA |
| Acetaldehyde | (38) | (39) | 69 | 21 | IA |
| Dimethyl sulfide | (36) | (38) | 99 | 37 | IA |
| Carbon disulfide | (22) | (30) | 115 | 46 | IB |
| Ethylene oxide | (20) | (29) | 55 | 13 | IA |
| n -Hexane | (7) | (22) | 156 | 69 | IB |
| Acetone | (4) | (20) | 56 | 133 | IA |
| Cyclohexane | (4) | (20) | 179 | 81 | IB |
| Tetrahydrofuran | 6 | (14) | 153 | 67 | IB |
| Benzene | 12 | (11) | 176 | 80 | IB |
| Triethylamine | 20 | (7) | 193 | 89 | IB |
| Methyl ethyl ketone (MEK) | 25 | (4) | 176 | 80 | IB |
| Toluene | 40 | 4 | 231 | 111 | IB |
| Methyl alcohol | 52 | 11 | 149 | 65 | IB |
| Isopropyl alcohol (IPA) | 53 | 12 | 180 | 82 | IB |
| Ethyl alcohol | 55 | 13 | 173 | 78 | IB |
| Pyridine | 68 | 20 | 239–241 | 116 | IB |
| 2-Nitropropane | 75 | 24 | 248 | 120 | IC |
| Tert butyl isocyanate | 80 | 27 | 185–187 | 85–86 | IC |
| Chlorobenzene | 82 | 28 | 270 | 132 | IC |
| Epichlorohydrin | 88 | 31 | 239–243 | 115–117 | IC |
| Xylene | 81–90 | 27–32 | 280–291 | 138–144 | IC |
| Morpholine | 100 | 38 | 263 | 128 | II |
| Acetic acid, glacial | 103 | 39 | 244 | 48 | II |
| Bromobenzene | 118 | 48 | 307–316 | 153–158 | II |
| Formic acid | 122 | 50 | 213 | 101 | II |
| Methyl lactate | 135 | 57 | 291 | 144 | II |
| Stoddard solvent | 100–140 | 38–60 | 300–400 | 150–200 | II |
| Iso -propyl lactate | 140 | 60 | 315 | 157 | II |
| Ethyl lactate | 142 | 61 | 307 | 153 | IIIA |
| Benzaldehyde | 145 | 63 | 352 | 178 | IIIA |
| Cyclohexanol | 154 | 68 | 322 | 161 | IIIA |
| Tetrahydronaphthalene | 160 | 71 | 406 | 208 | IIIA |
| Iso -butyl lactate | 169 | 76 | 360 | 182 | IIIB |
| Methacrylic acid | 170 | 77 | 316 | 158 | IIIA |
| Butyl lactate | 174 | 79 | 369 | 187 | IIIB |
| Nitrobenzene | 190 | 88 | 412 | 211 | IIIA |
| n -Methyl pyrrolidone | 199 | 93 | 396 | 202 | IIIA |
| Benzyl alcohol | 213 | 101 | 401 | 205 | IIIB |
| Caproic acid | 215 | 102 | 400 | 204 | IIIB |
| Ethylene glycol | 232 | 111 | 388 | 198 | IIIB |
| 3-Ethyllhexyl lactate | 235 | 113 | 475 | 246 | IIIB |
| Phenyl ether | 239 | 115 | 498 | 258 | IIIB |
| Stearic acid | 385 | 196 | 726 | 386 | IIIB |
Calculating Flash Points
*Measured by closed-cup method
Calculating Flash Points
The following formula allows for the calculation of flash points
1,000 / ( TF undefined + 273 ) = Bo + B1 · log10 · P25
TF = Flash point
Bo and B1 = constant (see table below) P25 = Vapor pressure of liquid at 25°C
This particular calculation is, unfortunately, not very accurate (variation with measured values can be as high as 100°C!) and requires the knowledge of the constants in Table 4-8 as well as the knowledge of the vapor pressure of the compound. Another more simplistic mathematical calculation starts with the autoignition temperature (AIT) in degrees Celsius of the compound . It is shown in Table 2.0 .
Table 3.0 . Constants for flash point calculations . (*These values produce more accurate results and were obtaned by excluding 2,2-dimethylbutane, naphthalene, dodecane, diphenylmethane, tetradecane, nonylbenzene, and decylbenzene.)
| Class |
Bo
|
B1
|
| Acetates |
2.976
|
0.380
|
| Acids |
2.777
|
0.491
|
| Alcohols |
2.953
|
0.323
|
| Phenols |
2.953
|
0.323
|
| Aldehydes |
2.924
|
0.443
|
| Alkanes |
3.142
|
0.319
|
| Alkanes* |
2.948
|
0.470
|
| Aromatics |
3.142
|
0.319
|
| Aromatics* |
2.948
|
0.470
|
| Alkenes |
3.097
|
0.424
|
| Amines |
3.077
|
0.322
|
| Esters |
2.948
|
0.385
|
| Ethers |
3.056
|
0.357
|
| Ketones |
3.033
|
0.381
|
Table 4.0 . Calculations of flash point .
| Class |
Flash point [°C]
|
| Paraffinic hydrocarbons and olefins in gaseous state at NTP |
350 – AIT
|
| Paraffinic hydrocarbons and olefins in liquid state at NTP |
250 – AIT
|
| Benzene series |
550 – (AIT + K)
|
| Alcohols (MW ≤ 60) |
8 + nHr
|
| Alcohols (60 < MW ≤ 88) |
11 + 2nHr
|
| Alcohols (MW > 88) |
29 + 3nHr
|
K = a variant (9 for each first branch CH3 and 21 for each second branch CH33 , 16 for each first branch CH and 12 for each second branch CH2)
nHr = number of hydrogen in radicals
Regulatory Requirements Related to Flash Point
Three U.S. government agencies and one private agency have requirements for users of cleaning solvents, based on the flash point of the solvent used. Again, these requirements have at least as much effect on the choice of cleaning solvent as does its solvency power.
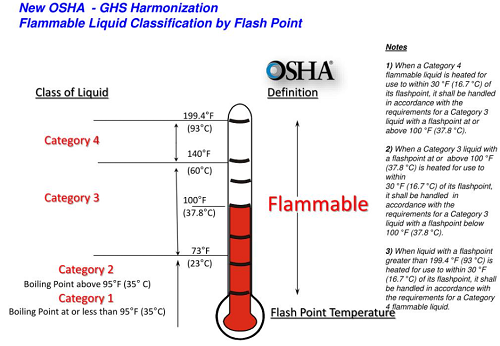
These requirements are defined in a classification system which is published in OSHA Standard 1910.106. 38
- Class IA—Flash Point less than 73 °F (22.7 °C); Boiling Point less than 100 °F (37.8 °C)
- Class IB—Flash Point less than 73 °F; Boiling Point equal to or greater than 100 °F
- Class IC—Flash Point equal to or greater than 73 °F, but less than 100 °F
- Class II—Flash Point equal to or greater than 100 ° F, but less than 140 °F (60 °C)
- Class IIIA—Flash Point equal to or greater than 140 °F, but less than 200 °F (93.3 °C)
- Class IIIB—Flash Point equal to or greater than 200 °F
Related:
- Flash Point and Fire Point of Oil
- Leidenfrost effect
- Fluids Lubrication Oil, Grease
- Synthetic Oils Review
- Naphthenic Oils Review
- Paraffinic Oils Review
References
- ASHRAE Handbook of Fundamentals, 2021 Inch-Pound Edition
- Flash Point Experimental Organic Chemistry, 2016
- OSHA Standard 1910.106