Related Resources: calculators
Thermal Molecular Velocity of Gas Molecules Formulas and Calculator
Fluid Dynamics Engineering and Design
Thermal Molecular Velocity of Gas Molecules Formulas and Calculator
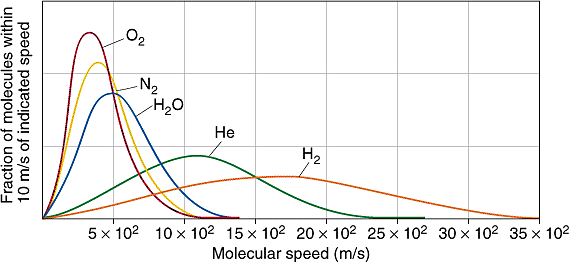
Gas molecules in a vessel move back and forth in different directions and at different speeds. Their velocity distribution corresponds to a bell curve having its peak at the most probable velocity
Probable Velocity
cw = [ ( 2 · R · T ) / M ]1/2
Mean Thermal Velocity
cmean = [ ( 8 · R · T ) / ( π · M ) ]1/2
Where:
cw = Probable Velocity (m/s),
cmean = Mean Velocity (m/s),
R = General gas constant R = 8.314510 kJ / (kmol K),
T = Thermodynamic temperature [K],
M = Molar mass [kg / kmol]
Molar masses and mean thermal velocities of various gases
| Gas |
Molar Mass (g/mol)
|
Mean Velocity (m/s)
|
Mach Number
|
| H2 |
2
|
1,762
|
5.3
|
| He |
4
|
1,246
|
3.7
|
| H2O |
18
|
587
|
1.8
|
| N2 |
28
|
471
|
1.4
|
| Air |
29
|
463
|
1.4
|
| Ar |
40
|
394
|
1.2
|
| CO2 |
44
|
376
|
1.1
|
Related:
- Converting scfm to acfm Equations and Calculator Flow Rate Measurement scfm (Standard Cubic Feet per Minute) and acfm (Actual Cubic Feet per Minute) Calculator
- Equivalence Table for Pressure/Vacuum Measurements (pressure reduction of 2 torr corresponds to 0.232% vacuum)
- Fundamentals of Vacuum Technology Dr. Walter Umrath, 200 Pages
- Knudsen Number (Kn) Equation and Calculator
- High Vacuum Technology fundamental properties of gases and vapors and their flow behavior
- Pressure Drop High Vacuum System Pipes per. Outlet Branch
- Pressure Loss Data for Sizing Vacuum Pipe, Low Pressure Vacuum System
- Time for Pump to Reach Rated Vacuum The time a given pump will take to reach its rated vacuum pressure depends on the volume of the system in cubic feet (cubic meters) and the capacity of the pump in scfm (sL/s) at the vacuum-rated pressure.
- Vacuum Pressure Units Conversion From Torr to Various Units Calculator
- Vacuum Mean Gas Velocity Equations and Calculator
- Vacuum Technology and Space Simulation, NASA, 315 Pages
- Vacuum Engineering Calculations and Applications
References:
- Karl Jousten (Hrsg.), Wutz Handbuch Vakuumtechnik,
- Pupp / Hartmann, Vakuumtechnik Carl Hanser Verlag
- Schweitzer, Bleuler, Traxler, Active Magnetic Bearings, Hochschulverlag AG an der ETH Zürich
- Fremerey, Vacuum 32 (1982) S.
- Hobson und Redhead, Can. J. Phys. 36 (1958)
- Redhead, Electron Stimulated Desorption, Vacuum 12 (1962)
- Dawson, Quadrupol Mass Spectrometry, Elsevier Amsterdam (1976)
- Brubaker, 14th National Symposium, Am. Vac. Soc. (1967)
- Fachbericht Balzers BG 800003 Das Funktionsprinzip des Quadrupol Massenspektrometers. (1990)